Let’s learn psychrometry & psychrometric chart. In this article, we will learn the basics of psychrometry & psychrometric chart, how to read & different parameters in the chart. Let’s explore!
What is Psychrometry? Definition & Meaning
Psychrometry Definition
The Psychrometry is the science of studying the properties of air-water mixtures. It is widely used to illustrate and analyze the characteristics of air-water mixtures in refrigeration, air conditioning processes, and many other processes.
Psychrometry Meaning
Do you know where from the word Psychrometry comes? Psychrometrics is a word which derived from the GREEK words, Psychro, and Metrics.
- Psychro means ‘to breathe’ or ‘to blow’ or ‘to make cold’
- Metrics means to measure
Hence, psychrometry is used to measure the properties of moist air, their control, the effect on materials, and human comfort. Moisture content in air varies significantly under different conditions like temperature, pressure, altitude, etc.
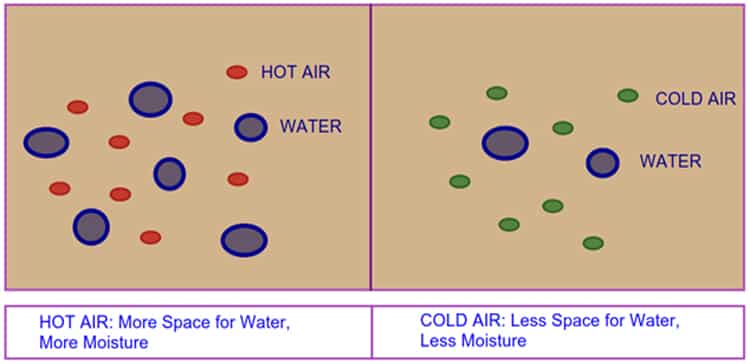
- Air can contain a large amount of moisture if it is hot.
- When the air is cold, it’s capacity to hold the moisture is reduced.
- When the temperature of hot air begins to reduce, the vapor also started to cool
- If cooling continues, air reduces to hold the capacity of containing moisture content and it will condense into moisture droplets.
What is Psychrometric Chart? Definition & Explanation
Psychrometric Chart Explanation
Psychrometric Chart is a graphical representation where all psychrometric parameters can be derived. Let us take an example when we are inside a car, sometimes we see water droplets outside the windows. Where from this water come? It comes from the surrounding air only. Moisture content present in the air comes in contact with the cold window surface & becomes small water droplets. Now, psychrometry is entirely defining all the properties of air-water mixtures.
What Does Psychrometric Chart Mean?
Psychrometry is developed into a psychrometric chart which has a wide function to understand the properties of air-water mixture and calculate the various parameters related to it.
- Any two parameters in the psychrometric chart can make the value of any other parameters.
- Easy to find out the condensate temperature.
- Easy to calculate the cooling or heating load requirements.
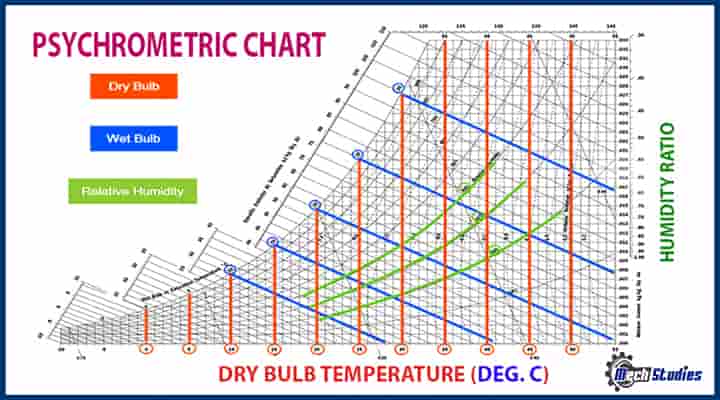
All the psychrometric chart is represented in a Psychrometric which provides the states or conditions of the air or water. A typical psychrometric chart is attached for reference. We all know, P-V diagram, this psychrometric chart is prepared by following three steps, refer to our video with animation.
What are the Basic Considerations for Psychrometry?
There are a few psychrometric considerations:
- Dry air and water vapor are present in the atmosphere.
- Both dry air and water vapor behave independently. Hence, we can easily consider dry air and water vapor separately as well as independently.
- When dry air is referred to, it will mean the air part;
- When water vapor or moisture is referred to, we will simply consider the water part only,
- When referring to just air, it will mean the mixture of dry air and water vapor.
- Complete dry air doesn’t exist, it always has some water vapor.
- Oxygen and nitrogen are the main part and the proportion of gasses do not vary, and it acts as a single gas. Hence, dry air is considered a single gas
- Dry air obeys the gas laws of Charles, Boyle, etc
- In the same way, water vapor also obeys gas laws.
In our rooms, the air is a mixture of dry air and water vapor.
Psychrometric Chart Terms & Definitions: Air, Vapor, Temperature, Humidity, Energy
All the psychrometric chart is represented in a Psychrometric which provides the states or condition of the air at any specific time. It displays the different properties of air:
- Dry-bulb temperature
- Wet-bulb temperature
- Relative humidity (RH)
- Humidity ratio
- Specific volume
- Dew point temperature
- Enthalpy
- Energy content
- Moisture content
- Specific volume and many more.
The Psychrometric terms are described with the explanation. If any of the two psychrometric terms are known, one intersection can be plotted and from that point, other parameters can be determined using the chart. Psychrometric Terms and definitions are defined below for a better understanding of the basics of air conditioning systems:
01. Psychrometry Terms for Air
Atmospheric Air: Atmospheric air contains the following:
- Nitrogen
- Oxygen
- Carbon dioxide
- Water vapour
- Other gases and miscellaneous contaminants such as dust, pollutants and smoke.
The amount of pollutants or water vapour varies based on the location. These pollutants, moisture content decrease with the height from the earth surface.

This is the air we breathe and use for air conditioning and ventilation systems. Hence, it is necessary to filtrate this air before using it.
Dry Air: Normal air consists of water vapor. The quantity of water vapor varies depending on the conditions. If water vapor and all impurities can be able to remove from the normal air, then dry air will be formed.
- The pure dry air does not exist in nature; it always contains some water vapor.
- Dry air is considered as perfect gas, as it exists in the atmosphere at low pressure.
- Water vapor is considered as perfect gas, as it exists in the atmosphere at low pressure.
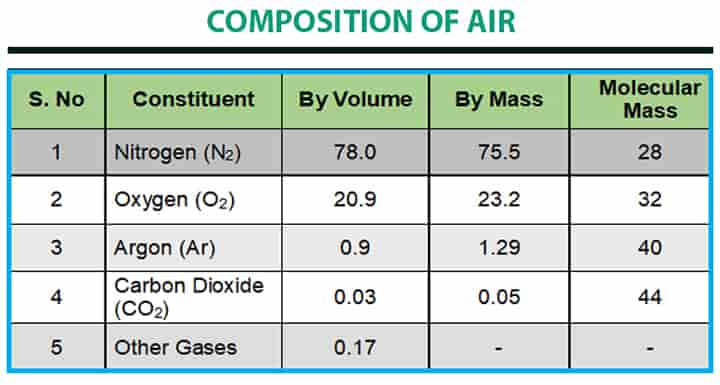
The dry air is considered to have the composition as given in the following table: There are some basic data of air, which is kept in mind:
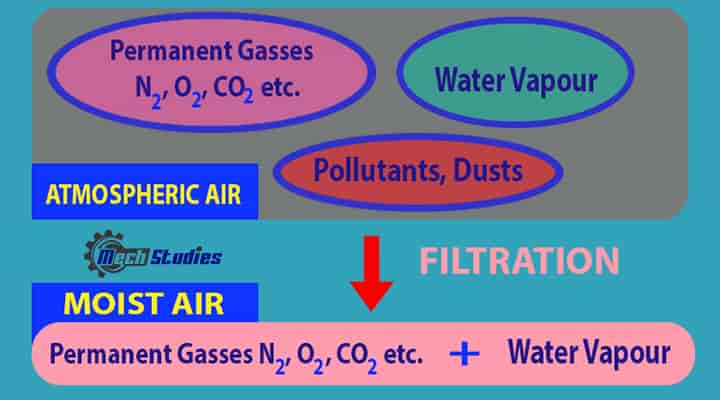
Moist Air: Moist means the presence of water. When moisture is present in the air, it is called as moist air. Moist air means a mixture of air and water.
- The moist air means a mixture of dry air and moisture or water vapor.
- The composition of dry air is fixed and changes are negligible. Hence, it can be considered as constant.
- The amount of water vapor present in the air depends upon the pressure and temperature of the mixture.
Unsaturated air: Air is always consisting of some amount of water vapor. This water vapor depends on pressure and temperature. Now we will take an enclosed space at some specific pressure and temperature and add moisture gradually. Initially, air consists in the enclosed space absorb the water vapour at the same pressure and temperature. This air is known as unsaturated air that means it can absorb or holds more water vapour.
Saturated air: But later, a stage will come when the air will not be able to absorb the moisture anymore. The extra moisture will be out in the form of dew or fog. The air which can hold the maximum water vapor or moisture at certain pressure and temperature is known as saturated air.
Saturated air and unsaturated air notes
- The maximum quantity of water vapor air can hold depends on the temperature.
- If the temperature of the air increases, air can hold more moisture,
- As per Dalton’s law of partial pressure, P = Pa + Pw, where Pa = partial pressure of dry air and Pw = partial pressure of water vapor.
- When the saturated air is cooled the water vapor starts to condense which is present in the air.
- Unsaturated air means air contains less amount of water vapor than saturated one.
02. Psychrometry Terms for Vapour
Let’s take some amount of water in an enclosed container and heat the water. Due to heating, the temperature of water gradually increases, and it will reach 100 deg. C. At 100 deg. C, the addition of heat also is not able to change the temperature of the water. Here, instead of temperature changes, water is started to convert into vapor.
- Unsaturated vapor: When the vapor is able to take the heat without temperature increase, it is known as unsaturated vapor.
- Saturated vapor: With further increase in temperature, the entire water will be converted to vapor. This vapor when all the water content is converted to vapor is known as a saturated vapor.
- Superheated vapor: If vapor is further heated, vapor will take the heat at 100 deg. C and there will not be any change in temperature. This steam which has more heat content than the saturated vapour is called superheated vapour.
03. Psychrometry Terms for Air Temperature
Dry Bulb Temperature
The Dry Bulb Temperature is simply the temperature of the ambient air that has no water on its surface. It is measured by normal thermometer & called “Dry Bulb Temperature” because the air temperature is indicated by a thermometer is not affected by the moisture of the air. When we talk about the temperature of the air, it is commonly referred to dry bulb temperature.
In the Psychrometric chart, Dry-bulb temperature is indicated on the X-axis, and all vertical lines in the chart represent constant temperature lines.
- It is denoted as td, tdb , Tdb or DBT etc.
- Dry-bulb temperature can be measured using a normal thermometer freely exposed to the air but shielded from radiation and moisture.
- The temperature is usually given in degrees Celsius (°C) or degrees Fahrenheit (°F).
- The SI unit is Kelvin (K). Zero Kelvin equals to -273oC
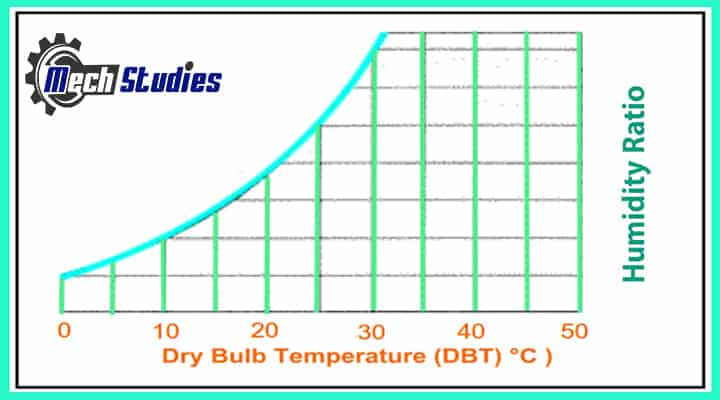
Measurement: You take a thermometer and hold it in the room and note the temperature. It’s basically the ambient temperature that you see, is known as ‘Dry Bulb Temperature’.
Wet Bulb Temperature
The Wet Bulb Temperature is simply the temperature of the air which is associated with moisture. It is measured by a normal thermometer & called “Wet Bulb Temperature” because the temperature is indicated by a thermometer is affected by the moisture of the air. When a normal thermometer is wrapped with a wet cloth, we get wet-bulb temperature.
- It is denoted as Twb, tw or twb
- Wet-bulb temperature can be measured using a normal thermometer wrapped with wet cloth.
- Wet-bulb temperatures are always lower than dry bulb temperatures and the only time that they will be the same is at saturation (i.e. 100% relative humidity).
- The temperature is usually given in degrees Celsius (°C) or degrees Fahrenheit (°F).
- The SI unit is Kelvin (K). Zero Kelvin equals to -273o
Measurement: You take a thermometer and hold it in the room wrapped with a wet cloth and note the temperature. It’s basically the wet-bulb temperature that you see, is known as ‘Wet Bulb Temperature’.
Note: Can you tell me when DBT and WBT both are same? Is it possible? Yes, normally it is different, however, check from the below graph that if the temperature is at saturation line, then both value will be the same.
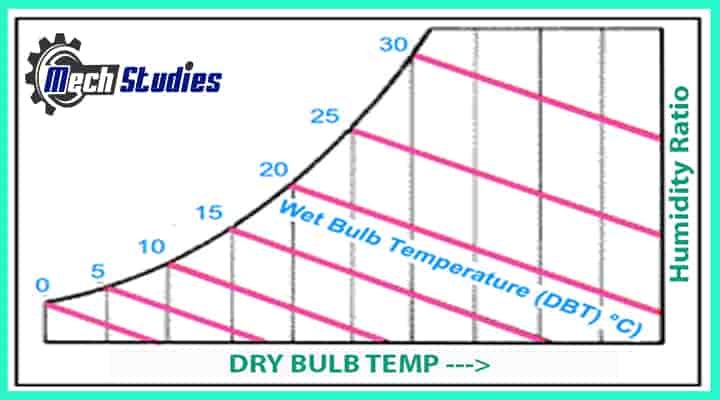
Dew Point Temperature
If we cool moist air, we will see that moist air will start to condense at a certain temperature. This temperature is called as dew point temperature. Hence, we can say, Dew point temperature is the temperature at which moisture present in the air starts to condense.
When air is cooled, the relative humidity increases gradually until saturation is reached, and after the saturation level, condensation occurs. Condensation occurs on surfaces that are at or below the dew point temperature.
- This is usually denoted by tdp or Tdp
- Dew point is represented along the 100% relative humidity line on the psychrometric chart.
- Dew point temperature is determined by moving from a state point horizontally to the left along constant humidity ratio up to curved saturation line.
- At dew point, dry bulb temperature and wet bulb temperature are the same.
- When the temperature of air reduces to the dew point, dew is formed.
- The temperature is fixed at the dew point temperature.
- The dew point temperature is directly related to the quantity of moisture in the air
- It is the saturation temperature corresponding to the partial pressure of water vapor
Wet Bulb Depression
Web bulb depression means simply the difference between the dry-bulb temperature and wet bulb temperature at any point. It indicates the relative humidity of the air.
Dew point Depression
This is the difference between the Dry bulb temperature and dew point temperature of the air.
04. Psychrometric Terms for Humidity
Relative Humidity
RH is an expression of the moisture content of a given atmosphere as a percentage of the saturation humidity at the same temperature. It is the ratio of an actual mass of water vapor in a given volume of moist air to the mass of water vapor in the same volume of saturated air at the same temperature and pressure. This is briefly written as RH or it can be written Φ as well.
- Φ = Pw/Pws = Moisture content at some pressure & temperature/Maximum moisture content to be held at saturation pressure & temperature x 100%
- Φ = Partial pressure of water vapor/Saturation pressure of pure water at that temperature x 100%
- Φ = Partial pressure of water vapor/vapor pressure of water vapor x 100%
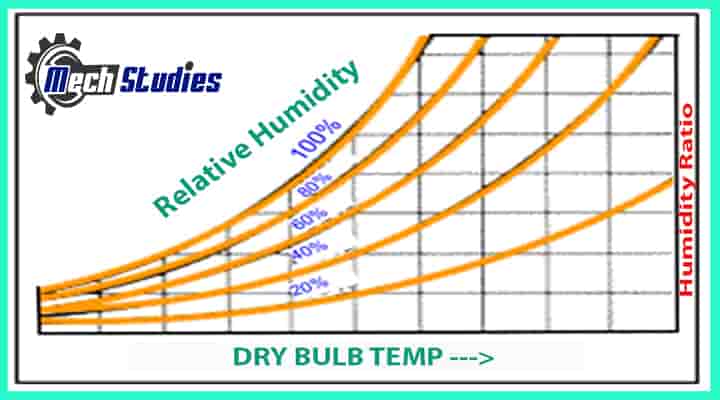
Absolute Humidity
The mass of water vapor per unit volume of dry air. For example, it can be written as the mass of water vapor in pounds per cubic feet of dry air, i.e. lbs/cu.ft of dry air. It is the mass of water vapor present in 1 m3 of dry air and is generally expressed in terms of a gram per cubic meter of dry air (g / m3 of dry air). 1 kg of water vapor is equal to 15430 grains.
Humidity Ratio & Specific Humidity
Humidity Ratio is the mass of water present in 1 kg of dry air and is generally expressed in terms of a gram per kg of dry air ( g/kg of dry air). However, when the ratio is between the mass of moisture and moist air, it is called specific humidity. As there is a negligible difference between humidity ratio and specific humidity, in the industry both are considered the same.
Saturation Humidity
Air at a given temperature can support only a certain amount of moisture and no more. This is known as saturation humidity.
Degree of Saturation
It is the ratio of an actual mass of water vapor in a unit mass of dry air (i.e., humidity ratio of moist air) to a mass of water vapor in the same mass of dry air when it is saturated (i.e., humidity ratio of saturated moist air) at the same temperature.

The degree of Saturation is expressed as; μ = x / xs , where
- μ = degree of saturation
- x = humidity ratio of the air
- xs = humidity ratio of the air at saturation at the same temperature and pressure
05. Terms for Energy
Enthalpy: Enthalpy is the measure of heat energy in the air due to sensible heat or latent heat. Sensible heat means the heat energy which changes the temperature of air and latent heat means the heat energy which cannot change the temperature. This sensible heat energy and latent heat energy are together known as enthalpy.
- Unit of Enthalpy is Btu per pound of dry air (Btu/lb of dry air) or kilojoules per kilogram (kJ/kg).
- Enthalpy is useful in air heating and air cooling applications.
Enthalpy Deviation: When we talk about enthalpy, it refers to the enthalpy of saturation. It should be corrected by the enthalpy deviation due to the air not being in the saturated state.
- The unit of Enthalpy deviation is Btu per pound of dry air.
- Enthalpy deviation is applied where extreme accuracy is required; however, on normal air conditioning, we don’t consider it.
06. Specific Air Volume
The cubic feet of the mixture per pound of dry air or cubic meter of the mixture per kg of dry air is represented in m3/kg. It is the reciprocal of density
07. Vapour Pressure
Latent heat is the heat content due to the presence of water vapor in the atmosphere. It is the heat, which was required to evaporate the given amount of moisture. Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapor state, and it increases with temperature.
08. Terms for Heat
Sensible heat: Sensible heat is one kind of heat that is the reason to increase the temperature of air i.e dry-bulb temperature of the air.
Latent heat: Latent heat is the heat content due to the presence of water vapor in the atmosphere. It is the heat, which was required to evaporate the given amount of moisture.
Brief Description of Psychrometric Processes
There are total 6 psychrometric process:
- Sensible cooling
- Sensible Heating
- Cooling and dehumidification
- Heating and Humidification
- Cooling & humidification
- Heating and de-humidification
01. Sensible Cooling
As air passes over a cooling coil, its moisture content remains constant while its temperature decreases. Cooling coils should have a dry surface and a surface temperature higher than the air’s dew point to maintain a constant moisture content. In the case of a 100% effective cooling coil, the temperature of the air at the exit will be equal to the temperature of the coil. In practice, however, exit air temperatures will be higher than cooling coil temperatures.
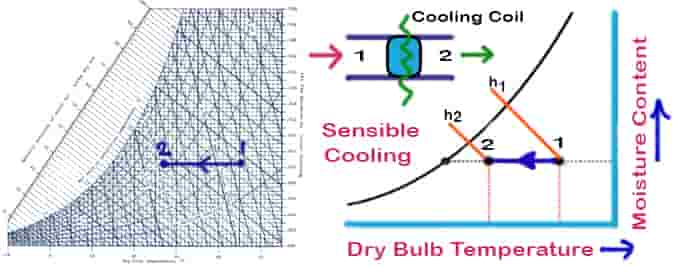
Things to remember for the sensible cooling psychrometric process:
- No change in moisture content
- Sensible cooling means the temperature will decrease
- The temperature line will be parallel to the DBT psychrometry reference line
The cooling load can be written as:
Qc = ma (h1 – h2 ) = ma cpm (T1 – T2 )
Sensible Heating
Sensible heating is the process of heating air without affecting its specific humidity. Allow air to be at the temperature at which it travels through a heating coil. The temperature of the air exiting the heating coil will be lower than the temperature, it should be noted. Air flows over a heating coil at a constant moisture content & temperature. Things to remember for the sensible heating psychrometric process:
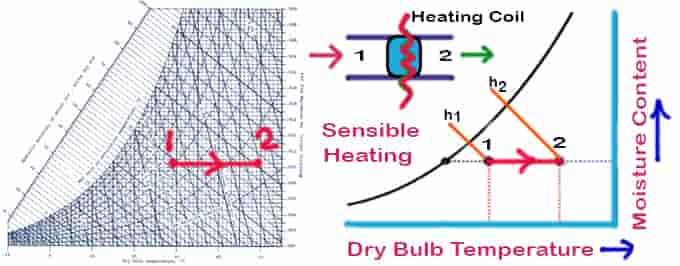
- No change in moisture content
- Sensible heating means the temperature will decrease
- The temperature line will be parallel to the DBT psychrometry reference line
Qh = ma (h2 − h1 ) = ma cpm (T2 − T1)
Cooling & Dehumidification
By cooling moist air below its dew point, some of the water vapor in the air condenses and leaves the air stream as a liquid, so both temperature and humidity ratio decrease as a result. This is how air is conditioned. For simplicity, the process line is assumed to be a straight line regardless of the type of cold surface, surface temperature, and flow conditions.
- ma .ωa = ma .ω2 + mw
- ma.ha = Qr + mw.hw + ma.h2
- Qr = ma (h1 − h2 ) − ma ( ω1 − ω2 )hw
- Qr = ma (h1 − h2 )
- Qr = Ql +Qs
- Ql = ma (h1 − hw ) = ma.hfg (ω1 − ωw )
- Qs = ma (hw − h2 ) = ma.cpm (T1 − T2 )
- Qs / Qt = Qs / (Qs + Ql )
Things to remember for the Cooling & Dehumidification psychrometric process:
- As the cooling process, the temperature will decrease.
- As dehumidification process, the moisture content will reduce
- The temperature, as well as moisture content, will be reduced, hence, this line will not be parallel to the DBT psychrometry reference line, it will be inclined and the direction will be on the lower side as per the diagram.
Heating and Humidification
To warm and humidify the air, the method is typically employed in winter air conditioning. It is the cooling and dehumidification process done in reverse. Unsaturated air will get saturated and become heated as it passes through a humidifier with spray water that is hotter than the dry bulb temperature of the air being introduced. The spray water itself absorbs the heat from the water vaporization process, which causes it to cool. This causes the air to warm and become more humid. In the winter season, the moisture content is reduced and the air becomes dry in many places except the sea coast area.
- mw = ma (Wd − Wo )
- Qh = ma (hd − ho ) – mw hw
Humidification & Cooling
The air temperature drops and its humidity increases as a result of this process. Cool water can be sprayed into the air stream to accomplish this. Ideally, the water temperature shouldn’t be higher than the dry bulb temperature of air, but it should not be lower than the dew-point temperature. In this case, the net transfer rate will be zero since the sensible heat transfer from air to water is equal to the latent heat transfer from water to air since the temperatures of water and air are comparable.
Heating & Dehumidification
A hygroscopic substance that absorbs or adsorbs the water vapor from the moisture can be used to carry out this procedure. The temperature of the air rises as the moisture content falls if the process is thermally isolated because the enthalpy of the gas remains constant. This hygroscopic substance can be either a liquid or a solid.
The absorption of water by hygroscopic materials often involves an exothermic reaction. As a result, heat is generated during this process, which is transmitted to the air and raises the enthalpy of air.
- mw = ma (ω2 − ω1)
- Qh = ma (h2 − h1) − mwhw
How to Read a Psychometric Table or Chart?
Here are a few points to help you read the psychometric table:-
- The varying temperature readings in Fahrenheit or Celsius are indicated on the chart’s horizontal, or “X” axis in figure 1. Trace the measurements throughout the chart using the vertical lines that branch off of this axis.
- A single pound or kilogram of moisture is represented as a vertical axis on the chart called the “Y” axis. In order to trace the humidity ratio all throughout the chart, you may use the horizontal lines extending from this axis.
- When the relative humidity is in hundred percent, this curve links the X and Y axes and shows the connection between temperature and absolute humidity. In keeping with this, take notice that the dew point and wet bulb temperature are both always the same as the dry bulb temperature.
- You will see several lines that resemble the saturation curve on the chart. These indicate the temperature-pressure connection when the relative humidity is below 100%. Most of the time, every line away from the saturation curve denotes a 10% reduction in concentration.
- Locate the line with the dew point measurement in degrees Fahrenheit or Celsius just to the right of the Y-axis in figure 2. Use a ruler to match the hash marks with the lines on the chart if you can’t see the lines on the graph.
According to the chart, the dew point does not fluctuate as a function of the dry bulb’s temperature.
- There will be marks that indicate the different vapor pressures in inches of mercury or millibars next to or along the same vertical line as the dew point readings. Once more, if you’re having difficulties following the chart’s lines, use a ruler to horizontally match the vapor pressures’ hash marks with the chart’s boundaries.
- This chart (Fig-2) is meant to measure the specific volume of air which is normally within a range of 2-3 m3/kg or ft3/lb, which is the volume of air within this chart that is meant to measure the volume of air. A given temperature increases the specific volume of air with increasing relative humidity.
What is Psychrometer?
A psychrometer is an instrument is used to measure the state of the air. It can easily measure,
- barometric pressure
- air dry-bulb temperature
Psychrometer consists –
- Two thermometers with the bulb are covered by a wet wick.
- Both the sensing bulbs are separated to avoid heat transfer between them
Two types of psychrometers are widely used:
- Sling psychrometer – measuring state where air velocity is less.
- Aspirated psychrometer – measuring the state of air for small fans or blower or ducts etc.
- Other types of psychrometric instruments:
- DPT meter
- Hygrometer (Using human hair or horse’s hair)
- Dunmore Electric Hygrometer
Application of Psychrometry & Psychrometric Chart
There are wide applications of psychrometry in our life as well as industries, like:
- Refrigeration industries.
- HVAC industries.
- All other industries related to refrigeration & air conditioning.
- Meteorological application etc.
Example of Psychrometry & Psychrometric Chart
In extreme summer, we use the air conditioning system to reduce the temperature of the air. Psychrometry helps us to control the temperature of the air as well as moisture content and makes us feel comfortable. We do all the calculations in the air conditioning system taking psychrometry as the primary concern.
FAQs on Psychrometry & Psychrometric Chart
What do you mean by psychrometry?
Even though the principles of psychrometrics can be applied to any physical system that contains a mixture of gasses or vapors. The most common system of interest is composed of water vapor and air since it is used in such applications as heating, ventilation, air conditioning, and meteorology. Human thermal well-being is determined both by the temperature of the surroundings and by how much water vapor is dissolved in those surroundings.
It is known that many substances are hygroscopic, which means that they are attracted to water, and this is usually proportional to relative humidity or above a critical relative humidity rate. Chemicals and fertilizers also belong to this class, as do cotton, paper, cellulose, and other wood products. Sugar, calcium oxide, and other substances are also included.
What is psychrometry in air conditioning?
Based on psychometric principles, cooling and heating systems are designed. The psychrometric chart is a diagram explaining the relationships between seven air properties, namely dry bulb temperature, wet bulb temperature, relative humidity, humidity ratio, specific volume, and many others, which can be immediately characterized under varying conditions.
What is psychrometry thermodynamics?
The study known as psychrometrics examines the thermodynamic characteristics of humid air and how to utilize them to examine humid air conditions and processes. Psychrometric and Mollier diagrams can be used to determine the processes involved in air conditioning.
What is a psychrometry and psychrometric chart?
Psychrometry
In psychrometry, the study of air is examined in terms of its many characteristics, methods for regulating its temperature and moisture content, and the effects it has on people and various materials.
It is possible to solve many natural and atmospheric riddles via the study of psychrometry by understanding the various components of air and how they interact.
Psychrometric Chart
Wet air is represented graphically, physically, and thermally through psychrometric charts. It may be highly beneficial in diagnosing and identifying solutions for environmental issues in livestock buildings or greenhouses.
Discernment of psychrometric charts can make it easier for you to comprehend topics related to environmental management, such as why hot air may store more moisture or, on other hand, how permitting wet air to cool would cause condensation.
What is psychometry in refrigeration?
The study of wet air’s thermodynamic characteristics, including its temperature, moisture content, enthalpy, and specific volume is known as psychrometry.
A portion of the moisture in the air that initially enters the cooler unit condenses on the coil. As a result, the air exits the device being colder and containing less moisture.
Even if the moisture content decreases, the relative humidity tends to rise as the temperature drops. This happens because cooling raises the density of the air and increases the amount of humidity in the vapor-air combination.
What does a psychrometric chart show?
A psychrometric chart condenses a lot of data into a graph with an unusual shape. The value of the chart will become more apparent if we look at each element separately. The dry-bulb thermometer gauge on the horizontal plane, the humidity ratio or moisture content scale on the y axis, and an upper curving border that denotes soaked air or adequate fluid holding capacity are the boundaries of the psychrometric chart.
Other significant moist air parameters are also displayed in the above chart, including wet-bulb temperature, enthalpy, dewpoint temperature or saturation temperature, relative humidity, and specific volume. In order to define moist air, any attribute’s intersection will be called a state point, and all other properties are readable from the state point.
What is a psychrometric chart in HVAC?
HVAC engineers and refrigeration engineers frequently use the Psychrometric Chart as a crucial tool in their work in the field of HVAC and refrigeration. A heat exchanger, a heat exchanger with a heat exchanger, an enthalpy disc, and an air handler can all be controlled by this system, which determines the heating and cooling costs of the building.
HVAC systems are designed using psychrometrics to maximize performance. Supply air streams must dissipate the same amount of sensible heat, and the same humidity ratio, in order to dissipate sufficient sensible and latent heat.
Which are the 5 factors indicated on a psychrometric chart?
Dry bulb temperature
Wet Bulb temperature
Relative Humidity
Dew Point temperature
Total Heat or Enthalpy
What is the importance of Psychrometry?
Here are a few benefits of having the knowledge of psychometry:-
By knowing the psychometric concept you can help yourself or utilize the knowledge for determining the properties of the atmosphere, which is a crucial step in the field of air conditioning.
Probably the most useful tool an air conditioning engineer or design technician has is a psychrometric chart.
The graphical examination of psychometric data and processes is made possible by the use of psychrometric charts.
The psychrometric chart offers solutions to a variety of real-world air-related issues.
What are the parameters of the psychrometric chart?
Psychrometric charts consist of a few standard parts, which are mentioned below:-
● Temperature
● Density:
● Enthalpy:
● Moisture content
● Relative humidity:
● Vapor Pressure:
What are the 6 psychrometric processes?
Sensible cooling
Sensible Heating
Cooling and dehumidification
Heating and Humidification
Cooling & humidification
Heating and de-humidification
Conclusion
So, we have learned the basics of psychrometry, psychrometric chart, and all related terms. We will be very happy if we get any feedback or suggestion to improve the post.
