Let’s learn about the types of metals. Metals are one of the abundant elements that are present on earth. This is one of the most prominent and essential things for humanity as it can be used for different purposes. Whether it is the construction or manufacturing of different equipment or structures, metals are used everywhere. It is even present in our water, which is one of the prominent presences of this material.
What are the Main Types of Metals?
Types of Metals Basics
Types of metals! Let’s start with the basics. While learning about metals, it is essential to get an understanding of the types of metal.
- The classification can be based on different attributes such as properties, extraction methods, applications, and many more.
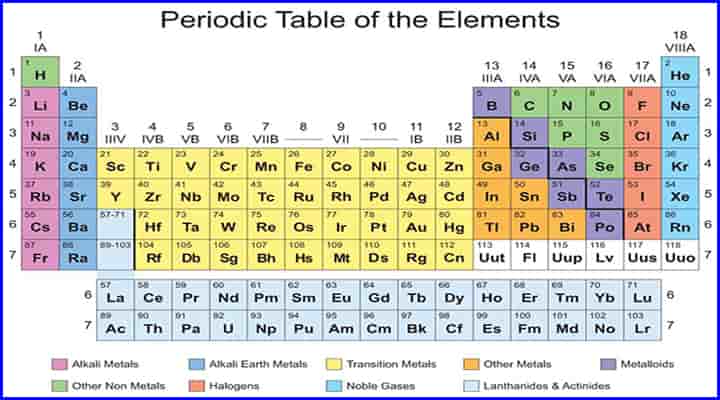
- One of the best ways to classify the materials is based on periodic tables.
- The periodic table consists of all the materials, including metals, non-metals, metalloids, etc.
Here we are providing information about types of metals based on classification under periodic tables.

Before delving into the types of metal, it is essential to get a brief idea about metal.
Metal Basics
Metals are the materials that have some specific properties such as,
- Malleability,
- Ductility,
- Lustrous and
- High density etc.
In addition to that, metals are also good conductors of electricity and heat. There are different kinds of metals available, and the periodic table consists of almost all of them.
Types of Metals
The classification of metals in periodic tables is in different types. The following are the types of metal subdivision:
- Alkali Metals
- Alkaline Earth Metals
- Transition Metals
- Other Metals
Description of Metal Types
The classification of metals in periodic tables is in different types. The following are the types of metal subdivision:
- Alkali Metals
- Alkaline Earth Metals
- Transition Metals
- Other Metals
Types of Metals #1 Alkali Metals
In the periodic table, the first column on the left side provides the classification of metals. These are soft metals and also highly reactive. The basic properties of alkali metal are its softness. These can easily be cut with any material. There are some metals in this category that even melt in the palm of your hand. These metals have low melting points. The six alkali metals are,
- Lithium
- Sodium
- Potassium
- Rubidium
- Caesium
- Francium

Lithium
| Properties | Highly reactive and forms robust hydroxide solutions. It is the only alkali metal that does not form the anion in solid-state or in any solution. |
| Application | The essential use of lithium is in metallurgical processes. It is used to refine some metals such as iron, nickel, copper, zinc, and their alloys. |
Sodium
| Properties | Sodium is a soft alkali metal that tarnishes when exposed to air. It reacts with water vigorously. |
| Application | The prominent use of sodium is in nuclear reactors as a heat exchanger. It is also used as a reagent in the chemical industry. The most common and abundant sodium use is in its compound form known as sodium chloride. It is added to food. |
Potassium
| Properties | The fundamental property of potassium is its rapid reaction with atmospheric oxygen. |
| Application | The core use of this metal is in the Medical field. It is specifically used as a medication to prevent and treat low blood potassium. Apart from that, it is also used for industrial purposes. |
Rubidium
| Properties | This is also a soft alkali metal that reacts violently with water and ignites in the air. |
| Application | The primary application of rubidium is in photocells. It acts as a vital component of photocells, which helps extract the oxygen traces from vacuum tubes. It is also used for making particular kinds of glass. |
Caesium
| Properties | Caesium is an alkali metal that has core properties that makes it soft. It is also very reactive to air and explosively reacts with water also. |
| Application | This metal is abundantly used in making special optical glasses, vacuum tubes, and also as a drilling fluid. The most crucial use of caesium is in caesium clocks, which are used in GPS satellites. |
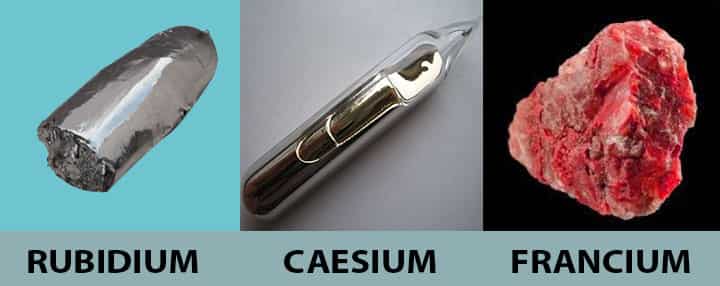
Francium
| Properties | This is one of the alkali metal that is intensely radioactive. The radioactive properties make it quite useful and lethal. |
| Application | When it comes to applications, it doesn’t have that much of life, making it not apt for wide usage. |
Types of Metals #2 Alkaline Earth Metals
The second column of the periodic table holds six types of metals known as alkaline earth metals. These are denser and harder than alkali metals. These metals hold a prevalent position among the metals group as the applications of these are quite vivid. The alkaline metals are as follows,
- Beryllium
- Magnesium
- Calcium
- Strontium
- Barium
- Radium

You check all types of metals in the periodic table . The following are the brief description of those alkaline earth metals:
. The following are the brief description of those alkaline earth metals:
Beryllium
| Properties | This is one of the highly toxic elements. This alkaline earth metal is among those light metals which have high melting points. These metals exist in different kinds of minerals. The excellent thermal conductivity is among the foremost properties that can be used for different purposes. |
| Application | Beryllium is used widely in different prominent fields. One of the areas that use this metal is the defense aerospace industry. It is also used as an alloying agent. Among all the applications they use in the making, some of the computer peripherals make it quite popular among all metals. |
Magnesium
| Properties | The foremost property of this metal is its chemical activeness. It is among the eighth-most abundant metals. The different essential properties make it quite useful for different kinds of usage. |
| Application | It is widely used for medicinal purposes. With this, one can easily cure different medical conditions such as anxiety, attention deficit-hyperactivity disorder (ADHD), anxiety, recovery after surgery, etc. |
Calcium
| Properties | Calcium is among the most abundant metals in the planet’s crust. It is equipped with those efficient properties that help in different biological advancements. |
| Application | The main applications of calcium are for bones and teeth. It also allows in our biological functionalities. Apart from these, the abundant usage of this metal is in the food and pharmaceutical industry. |
Strontium
| Properties | This metal is present in all living organisms, so it holds those properties that make it quite vital. Preferably it is located in the bones. The properties of strontium and the amount of this metal defines the benefits and loss. |
| Application | The main application of strontium is to make prosthetics and provide biological support to different parts of the body. |
Barium
| Properties | The basic properties of this metal is its lightweight and low density. It oxidizes with air and reacts with water. It can react with almost all the non-metals that make it lethal, and it even forms poisonous compounds. |
| Application | The main application of barium is in vacuum tubes and spark plug electrodes. Apart from that, it is also used as fluorescent lamps. The barium compounds are also used for the manufacturing of bricks, tiles, glass, making paint, and rubber. |
Radium
| Properties | The main properties of this metal are its high reactiveness with air. It is amongst the heaviest alkaline earth metal, which has radioactive properties. |
| Application | The practical use of this metal is in instrument dials, nuclear panels, self-luminous paints for watches, aircraft switches, and clocks. It is also used in medicine that helps in the treatment of cancer. |
Types of Metals #4 Transition Metals
The transition metals are found in the center of the main body of the Periodic Table. They are sometimes called heavy metals and are denser than alkali or alkaline earth metals. In total, there are 38 different types of transition metals available. Here we are providing some of the essential ones that hold specific places, like,
- Cobalt
- Copper
- Gold
- Iron
- Mercury
- Platinum
- Tungsten
- Zine etc.

Let’s know the basics of these types of metals,
Cobalt
| Properties | The core properties of this metal are its lustrous, brittleness, and ferromagnetic ones. It remains stable in the air and doesn’t react with water. This metal has magnetic properties. With diluted acids, it reacts at a gradual pace. |
| Applications | It is used in different magnets and magnetic recording media. It is used as a drying agent for inks and paints. The primary application of this metal is for artisans who work with enamel jeweler or porcelain etc., and it has medical usage. |
Copper
| Properties | Copper has one of the prominent properties that keep it away from corrosion. The alloying properties make it suitable for use in different types of alloys. |
| Applications | The prominent application of copper is in different electric peripherals and pieces of equipment such as, TVs, DVD players, iPods, electric kettles, mobiles, vacuum cleaners, computers, cars, washing machines, fridges, buses, electrified railways, underground transport systems, or trams. |
Gold
| Properties | Gold is among the densest metals. It is one of the valuable metals that holds massive importance. It is a soft, malleable, and ductile metal. Apart from that, it is among those metals which is a good conductor of heat and electricity. |
| Applications | The foremost use of gold is in the formation of jewelry. It holds a special place in the life of every human being. |
Iron
| Properties | This metal is equipped with numerous properties that make it quite incredible. These properties make it metal with maximum applications. It has the ferromagnetic ability, malleable, dissolvable. This metal is also a good conductor of heat and electricity. |
| Applications | There are different kinds of applications of this metal. It is specifically used for the manufacturing of tools and weapons. Apart from these heavy structures, and construction also uses this metal. Without this metal, you cannot imagine the present world. |
Mercury
| Properties | This metal has some specific properties that make it unique. It is a volatile liquid. This metal’s physical property is quite significant as it has very high surface tension, and it is also an excellent conductor of electricity. When it comes to chemical properties, this metal is moderately active as it doesn’t react with oxygen in the air. |
| Applications | Mercury has wide application as it is used in different measuring instruments such as, float valves, mercury switches, thermometers, barometers, manometers, sphygmomanometers, mercury relays, fluorescent lamps due to its high density. |

Platinum
| Properties | Platinum is among the valuable materials that are soft and lustrous. It has high density, ductile and malleable. Other than that, it also has a high boiling point and corrosion-resistant properties. It is amongst the most stable metals in nature. |
| Applications | Platinum is used in Optical fibers, wires, and pacemakers. It is also used as a catalyst for chemical reactions. Apart from that, it is also used for making jewelry. |
Tungsten
| Properties | Tungsten is a metal that has the highest tensile strength. It remains brittle at room temperature. One of the primary uses of this tungsten is due to its withstanding capability to high temperature. |
| Applications | Due to this metal’s properties, it is used in incandescent and fluorescent light bulb filaments. It is among the most primary metals that are used in television tubes. |
Zinc
| Properties | Zinc is very sparkly and attains a whitish-blue color. It is comparatively harder than copper. It is an essential trace element in the human body. |
| Applications | The core usage of this metal is vivid. It is used in the manufacturing of roofing materials as well as in solar cells and nuclear cells. Due to the core property of withstanding high temperature, it is used to make automobile tires. |
Types of Metals #5 Other Metals
Different other metals lie on the right side of the periodic table, either semimetals or metalloids. These are also known as post-transition metals. The core attributes of these metals are their softness and lower melting points. When it comes to usage, these metals are equipped with core properties that make them suitable for different kinds of applications. Some of the examples of these metals are as follows:
Aluminum
| Properties | The core properties of aluminum are its softness. It is highly ductile and malleable, which makes it apt for rolling into a thin foil and can be drawn into wire. It is a chemically active metal that makes it quite useful. |
| Applications | This is one of the best elements that is used in numerous alloys. The effective use of aluminum is aircraft construction, food-processing equipment, building materials, refrigerators, air conditioners, cooking utensils, electrical conductors, and chemicals. |
Lead
| Properties | The core properties of this metal are its toxicity. It increases solubility. The poisonous effect of lead can be very lethal as it causes different diseases. Apart from these, it has nuclear properties that help in different purposes. |
| Applications | The wide usage of this metal is in the construction of batteries. This is among the best uses as humankind is virtually dependent upon the batteries, which are part of everything. It is also used as protective shielding around nuclear reactors, X-ray equipment, and particle accelerators. |
Tin
| Properties | Tin is among those metals with the core features of malleability, ductility, and a non-toxic approach. This is also among those metals that are widely accepted for different purposes. |
| Applications | The tin’s main applications are in piping and a constituent for iron alloys due to its non-corrosive properties. The massive use of this metal is in the food industry, where it is widely used as a container for the same. |
What are the Most Common Five Metals?
With thousands of types of metal and just as many grades, there is no limit to the possibility of applications and uses that can be found only by the limits of our imagination. The metals have played a very important role in the development and growth of not just our society but also the development and growth of civilization as a whole during the past few centuries as a foundation for many industries and at the core of the Industrial Revolution.
Among the most popular types of metal that are used throughout the world are the following five types. Throughout this article, we have explored some of the infinite uses of these metals and provided some insight into the specific types of metals used.
Iron
A highly abundant metal, iron makes up about 5% of the Earth’s crust and is one of the six most common elements in the universe, making it one of the most popular metals we have today. As an unstable element, unalloyed iron readily reacts with the oxygen in the air to form iron oxide when it comes into contact with such a reaction. The use of steel is primarily due to the alloying of iron with other elements, resulting in a more
The porous surface of iron is a great choice for cookware because it prevents food from sticking to it when combined with hot oil. There is a reason why cast iron is used as a material for making wood stoves, which is due to its exceptionally high melting point. In addition to being a heavy metal, iron provides rigidity as well as reduces vibration, which is why it is often used for making heavy machinery frames and bases due to its rigidity and vibration-reducing properties.
Steel
It is an iron alloy that is enriched with around 1 percent carbon, and it has the advantage of being free of the impurities and residues that can appear in iron if it was not enriched with carbon. Despite its heavy, dense, corrosive, and heavy nature, iron is one of the strongest metals in the sense that it is much more durable than anything else. This is one of the reasons why purely iron structures have a difficult time being constructed and kept in good condition. This vulnerability is partly mitigated by the addition of carbon to iron, but it is also strengthened by the addition of carbon.
Aluminium
There are several attributes that make aluminium a very durable, light-weight, and corrosion-resistant metal that comes from its ore, bauxite. Due to its machinability, electrical conductivity, and inability to magnetize, aluminium was found in widespread use throughout the 19th century, making it one of the most widely used materials. A common non-ferrous metal on Earth, titanium is known for its malleability and the ability to form alloys with almost any other metal, which makes it the most common non-ferrous metal in the world.
In terms of strength to weight, aluminium has one of the highest ratios of all the metals, and it does not rust when exposed to salt, but when it is exposed to salt, it cannot resist corroding and oxidizing, which occurs when it is exposed to salt. This type of material finds application in a wide range of products: from household appliances to cans and aero planes.
Zinc
A common metal that has a low melting point is zinc, which is a common metal. It has the advantage of flowing smoothly in its melted form, and because of this, it is easy to cast and recycle. It is quite a strong and electrochemically inert end product, and it has a low electrochemical potential. As well as being used for coating and protecting other metals, zinc is also used in the manufacture of galvanized steel to prevent rusting. In addition to its industrial, marine, and medical applications, it can also be used in the hardware, electrical, and automotive industries.
Bronze
As a result of alloying copper with tin, bronze typically contains small amounts of lightweight elements like aluminium, manganese, silicon and phosphorus in addition to copper and tin. Because of the fact that bronze is the first alloy to be manufactured by humans, it is incredibly historical – thus the term “Bronze Age.”
Even though bronze is hard, it is known to be a material that resists fatigue and does not crack or bend. It is used in a wide range of applications, such as the production of electrical connectors, church bells, ship parts, and reflectors, due to its high corrosion resistance and solid thermal and electrical conductivity.
What Types of Metal are Magnetic?
A piece of metal will be magnetic if the crystalline structure of the atoms is aligned in the same direction. There is no pattern to the alignment of the atoms in most materials. Magnetization is only possible with ferromagnetic materials. Ferromagnetic metals usually do not have aligned atoms in their natural state, so they must be magnetized. You can magnetize metal to create permanent magnets, temporary magnets, or electromagnets by magnetizing it
Permanent Magnets
Permanent magnets are metals with permanently aligned crystalline structures.
Temporary Magnets
Metals that create a magnetic field under certain conditions are temporary magnets.
Electro magnets
Wires are coiled around ferromagnetic materials and electrical current is run through them to create electromagnets. In this type of magnet, the magnetic field only exists while the electrical current flows. Relationships like this run both ways, as well. An electrical current can be created by moving a magnet through a coiled wire.
Many common metals, including aluminium, copper, brass, gold, silver, titanium, tungsten, and lead, are non-ferromagnetic. Magnetic fields will not attract them, and they cannot be made into magnets. Metals containing iron are magnetic, so they will be attracted to magnets. Steel paper clips are attracted to magnets as well, since steel contains iron.
What are the Four Main Categories of Metal?
Carbon steel, stainless steel, alloy steel, and tool steel are the four main types of metal.
Carbon Steel
The name carbon steel implies that it is a special type of steel with a high concentration of carbon. There is a relatively low carbon content in most types of steel, between 0.05 percent and 0.3 percent. However, carbon steel is more prone to containing more than 2.5 percent carbon. There are several benefits to two-and-a-half percent carbon that are not available anywhere else.
Merits
Strength is one of the many advantages of carbon steel over traditional steel. By shuffling around its crystal lattice, carbon strengthens iron or steel. Even though carbon steel can still break and stress under tension, this is significantly less likely to happen than other steel types. In applications where strength is required, carbon steel is particularly useful. Many centuries ago, Japanese blade-smiths produced swords made from high-carbon steel called tamahagane steel.
From construction materials to tools to automotive components, carbon steel is used in a wide range of applications today.
Demerits
While carbon steel offers some advantages over traditional steel, there are also some disadvantages. It’s hard to work with carbon steel because it is so strong. Certain applications are limited by its inability to bend and mold into different shapes. Compared to other types of steel, carbon steel is more susceptible to corrosion and rust. About 10 percent to 12 percent of chromium is added to steel to make it stainless. Over the steel itself, chrome acts as a barrier of protection against moisture that may otherwise cause rust. When exposed to moisture for a long time, carbon steel may rust since it does not contain chromium.
Stainless Steel
Alloys containing iron and chromium make up stainless steel. It is mandatory that stainless steel contain at least 10.5% chromium, but the exact components and ratios will vary based on the grade requested and how it will be used.
Merits
Corrosion resistance is the primary purpose of stainless steel manufacturing. A small barrier protects the steel surface as it corrodes due to the addition of chromium to the iron & carbon ratio. Many high-contact appliances and tools, such as surgical instruments and cutlery, are made of stainless steel.
Alloy Steel
Steel alloyed with more than one alloying element is called an alloy steel. The purpose of adding these elements is to increase the strength, hardness, wear resistance, and toughness of the material. A typical alloy steel’s material composition consists of no more than 5% alloying elements added to the iron and carbon base.
Merits
There is alloy steel that provides the corrosion resistance, machinability, strength, or any other feature you require. When alloy steels are heat treated, they can provide a wide range of beneficial properties, such as enhanced corrosion resistance, increased harden-ability, and superior strength and hardness.
Tool Steel
Known for their hardness, wear resistance, toughness, and resistance to softening at high temperatures, tool steels are a family of carbon and alloy steels. Chromium, vanadium, molybdenum, and tungsten are carbide-forming elements in tool steel.
Conclusion
This excerpt is beneficial for those who want to get a holistic idea of the different metals available on earth. However, this classification is based on metals present in the periodic table, while there are other differentiating properties on which these metals can be classified.
The above excerpt specifically holds the core properties and the significant applications of these metals, which will help understand these metals. Apart from the above types of metals, some others have massive importance, such as rare earth metals, lanthanides, and actinides.
FAQs on Types of Metals
What types of metal are not magnetic?
There are a number of other metals that are non-magnetic, such as aluminium, copper, and gold. Gold and silver are the only metals that are not magnetic.
What are the main types of Metal?
Ferrous metals contain iron, while non-ferrous metals do not. Metals can be divided into two main categories based on their iron content: ferrous and non-ferrous.
What are the 3 types of Metal?
In an extremely large-scale industrial process, metal is extracted from the rocks. In general, metals can be divided into ferrous, nonferrous, and alloys.
How many kinds of metal are there?
Metals are classified in different ways on the periodic table, according to the Royal Society of Chemistry.
What is the cheapest metal out of all the metals?
There are many metals on the planet, but aluminium is the most plentiful and cheapest metal on the planet. Besides being one of the most abundant metals in the Earth’s crust, it also has a very good connection with other substances since it forms strong bonds with them.
What type of metal is considered the strongest metal?
Hardness can be divided into several categories in terms of metal strength, and some of these categories include yield strength, compressive strength, and tensile strength. In addition to measuring a metal’s overall strength, these standards also provide an accurate measure of the quality of the metal itself.
A number of metals such as chromium, tungsten, and titanium, which are commonly found in nature, present excellent strength characteristics, but in comparison to man-made metals such as steel and Inconel alloys, they are not as intense as natural metals.
In terms of strength, tungsten is rated as the world’s strongest metal, which is derived from the Swedish word “heavy stone”.
Which metal are stronger: magnetic or non-magnetic?
There is no relationship between a metal’s magnetic properties and its strength or weakness. Steel, for example, is one of the strongest ferromagnetic metals. The ferromagnetic properties of titanium and other strong metals can be varied.
Soft magnets are also a type of magnet. An annealed steel or iron is one that has been chemically or physically altered to reduce its hardness. Many of the softest metals we know, including lead, gold, and tin, are not magnetic.
What type of metal is gold?
Gold is a transition metal is an element that belongs to group 11 and is chemically considered to be a transition metal. It is a chemical element that is considered to be one of the least reactive, and it is solid under standard conditions. Alluvial deposits and igneous rocks are typically found with native gold in the form of nuggets or grains, which are often found in the free elemental or native form.
Can metal detectors detect non-magnetic metal?
Non-magnetic metals such as gold, silver, copper, and tin can be detected by metal detectors. The only materials that are magnetic are those that are ferromagnetic, such as iron, cobalt, and nickel. Ferromagnetic, Paramagnetic, and Diamagnetic are three categories of metals.
It is impossible to detect diamagnetic or paramagnetic metals with normal human senses but metal detectors can detect them.

Very interesting subject , regards for posting.