What is thermodynamics, or thermodynamics is the most common term in engineering, will be explained in this article. We will learn, the basic, definition, different branches, properties, etc. with a simple explanation. Let’s Explore!
What is Thermodynamics? Definition & Meaning
What Does Thermodynamics Mean?
The word, Thermodynamics came from the Greek word THERME and DYNAMICS.
- THERME means HEAT in GREEK.
- DYNAMICS means POWER in GREEK.
Hence, we can say, thermodynamics is related to the heat and the power or the energy. So, thermodynamics means,
- Heat or temperature
- Work
- Power or energy
- The relation between heat, work & energy.

Electricity is produced by different kinds of power plants, like, thermal power plant, nuclear power plant, etc. Do you know how power plants produce power or electricity? We get electricity from power plants but do you know how do these power plants produce electricity?
- The power plant uses fuel, as the fuel has stored thermal energy.
- This thermal energy is converted into power or electricity in power plants.
- This is a simple example of thermodynamics.
We take food to get the required calories. How do we receive these calories? It is because each food has stored energy which we take.
Thermodynamics Definition
In simple language, thermodynamics is basically a science, which is related to heat, work, and properties related to heat and work. Hence, it is defined as:
Thermodynamics is a science deal with heat and work and the properties related to heat and work.
– P. K. Nag.
Thermodynamics can be explained, as,
- Study of energy
- Energy conversions or energy transformation
- Study of heat, work
- Relation to matters.
- The main three parameters are Heat, Work & Energy in thermodynamics.
- It deals with the relationships between heat, work & energy.
- Thermodynamics has many processes
- It is necessary to describe power generation systems, refrigeration systems, HVAC systems, combustion processes, fluid flow, etc.
Thermodynamic Approach
There are two approaches of thermodynamics by which we can study the behavior of matters:
- Macroscopic approach, macro means big or total or average
- Microscopic approach, micro means very small
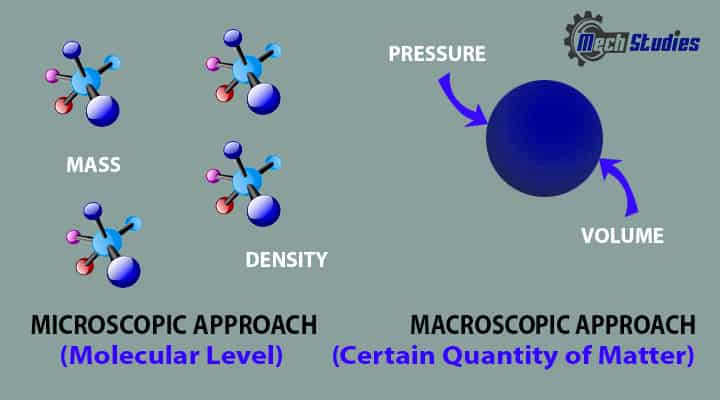
Macroscopic Approach in Thermodynamics
In the macroscopic approach or it is known as the classical approach, we concentrate a certain quantity of matter without considering the activities happening at the molecular level.
- In this approach, we determine properties like Pressure, Temperature, Volume, etc. which normally get affected by the systems interact with the surrounding.
- Macroscopic matters consider only a certain quantity of matters without consideration of any molecular level.
- The structure of matter is not considered.
- It doesn’t require any knowledge of individual molecules.
- In this case, the effects of many molecules can get and that could be sensed by human beings. There are macroscopic properties that cannot be sensed by human beings that can be related to the properties which may be sensed by humans beings.
- Observations are totally independent of the nature of matters
- In a macroscopic view, a bulk of molecules or a collection of molecules has been considered as a single component.
- Variable can be measured by instrument directly or indirectly.
Microscopic Approach in Thermodynamics
In a microscopic view or known as a statistical approach, we try to determine the behavior of a system by the events happening at the molecular level.
We can easily find that macroscopic behavior is always related to microscopic behavior because the matter is always comprised of molecules. The microscopic view has the following characteristics:
- Microscopic matters consider myriads of molecules and action is based on the same.
- It’s a study of individual molecules and requires any knowledge of individual molecules.
- In this case, the effects of many molecules can get but that could not be sensed by human beings.
- In the case of gas, each molecule has a specific velocity, energy, and position.
- Observations are totally dependent on the nature of matters
- The microscopic view, each molecule has been considered as a single component.
- Variable cannot be measured by instruments directly or indirectly.
What are the Branches of Thermodynamics?
Thermodynamics has different branches, which is simply the classification as well. These are as follows,
- Classical Thermodynamics
- Statistical Thermodynamics
- Chemical Thermodynamics
- Equilibrium Thermodynamics
Classical Thermodynamics
The classical thermodynamics means,
- In this kid of thermodynamics, the macroscopic approach is used to analyze the behavior of matter.
- Pressure, temperature, etc. are considered to calculate the other properties as well as characteristics
- The exchange of heat, work, and energy is studied broadly.
- Classical thermodynamics is used to understand the basic level of the behavior of matter.
Statistical Thermodynamics
- In the statistical thermodynamics, atomic or molecule levels are considered.
- Basically, the macroscopic approach is considered.
- The behavior of molecules can be analyzed.
- It is supplemented classical thermodynamics.
Chemical Thermodynamics
- It deals with the study of energy or energy interaction with chemical reactions.
- In the chemical reactions, it relates to heat and works along with the change of states.
Equilibrium Thermodynamics
- It is the study of energy transfer or matter transfer with surroundings.
- The study is due to the approach towards the equilibrium state.
- Thermodynamic equilibrium’ means there will not be any flow between the system and the surrounding.
- There will not be any unbalanced forces.
- In addition to equilibrium thermodynamics, there is Non-equilibrium thermodynamics
- In non-equilibrium thermodynamics, the system is not in equilibrium with surrounding.
- Unbalanced forces are present.
- These are not stable.
Thermodynamic Basics Terms & Explanation
To understand what is thermodynamics, we have to learn a few basics of thermodynamics so that a nice concept can be built. Thermodynamic terms are,
- System, Boundary, Surrounding & Universe
- Thermodynamic Systems
- Thermodynamic Process
- Thermodynamic Properties
- Thermodynamic state
- Thermodynamic equilibrium
- Internal energy
- Enthalpy
- Entropy
Let us learn these all, in brief
System, Boundary, Surrounding & Universe
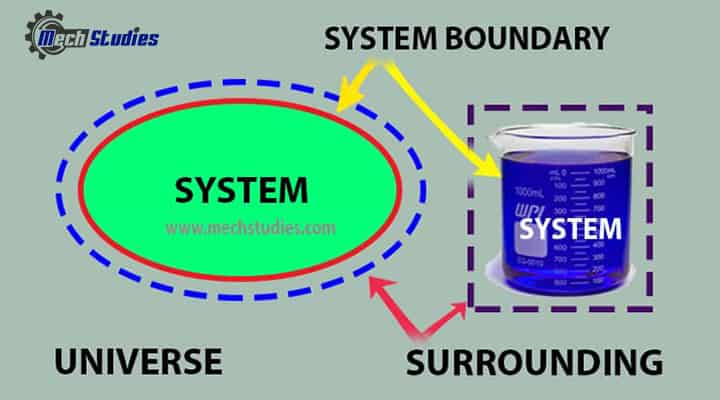
System
- Space where properties or processes are measured or analyzed.
Boundary
- It is the real or imaginary surface that bounds the system.
- It differentiates the system from its surroundings.
- It can be fixed or movable.
Surroundings
- Everything external to the system is called as the surrounding.
- It means outside of the system.
Universe
- The universe means the combination of system and surroundings.
- It is simply written as, System + Surrounding = Universe.
Thermodynamic Systems
There are two kinds of exchange happens between the thermodynamic system and surroundings,
- Mass exchange
- Energy exchange
Based on the above, the thermodynamic system is divided into three types,
- Open system
- Closed system
- Isolated system.
Open System
Open system means open where mass or energy can transfer across its system boundary.
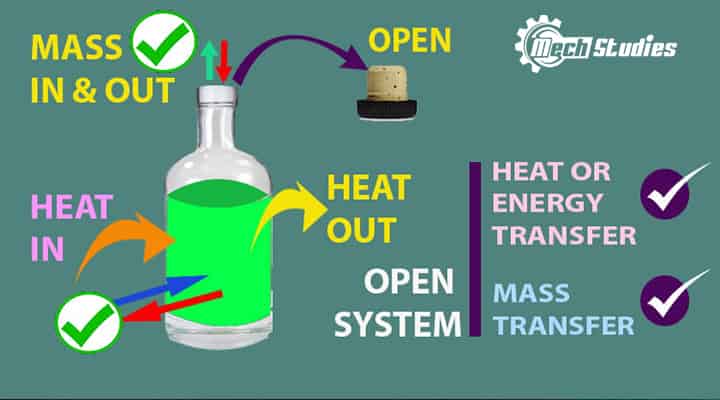
It means,
- It is open to the surroundings
- Mass transfer can happen across the boundary.
- Mass can in or out of the system.
- Energy transfer can happen across the boundary.
- The energy in or out of the system.
- Mass and energy can transfer together as well.
- It is also called a control volume.
- Boiling water is an open container is an example of an open system.
Closed System
It is closed or separated from the surrounding by a physical boundary.
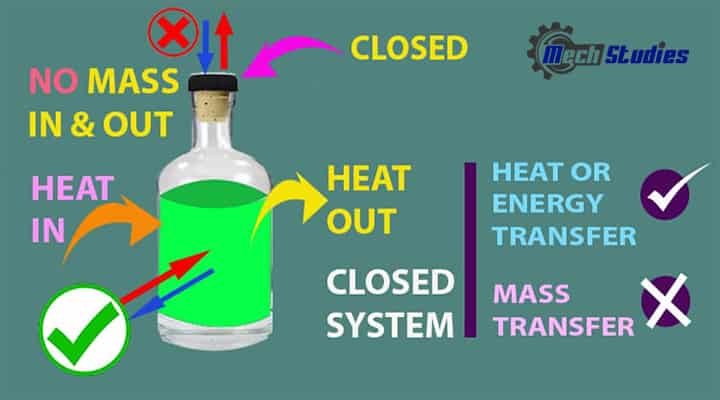
It means,
- As it is closed, mass cannot transfer.
- Energy transfer happens through the boundary, as the system is not insulated.
- Boiling water in a pressure cooker is an example of a closed system.
Isolated System
An isolated system means the system is totally isolated from the surrounding.
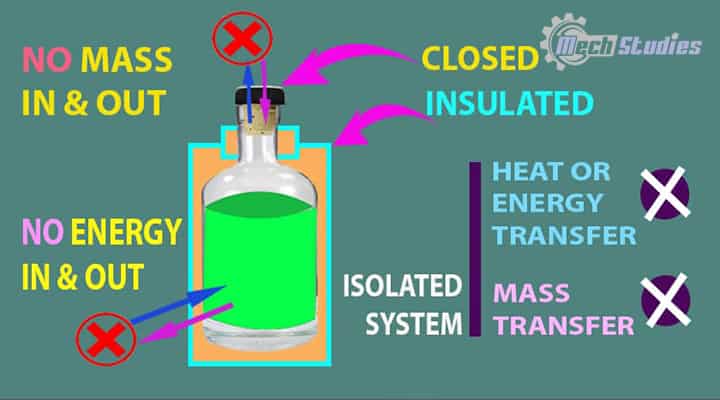
It means,
- No interaction with surrounding,
- No mass transfer happens.
- No energy transfer happens.
- Thermos Flask is an example of an isolated system.
Thermodynamic Process
Any change of a system, when it undergoes from one equilibrium state to another equilibrium state is known as a process. There are different kinds of processes, based on the changes in energy, pressure, volume, etc.
Isobaric Process
- It is the thermodynamic process, in which there will not be any change in pressure.
Isochoric Process
- It is the process, where no change in volume happens.
- The system is not able to work.
Isothermal Process
- In this process, there will not be any change in temperature.
Adiabatic Process
- The adiabatic process
 means, there will not be any heat transfer into the system or out of the system.
means, there will not be any heat transfer into the system or out of the system.
Reversible process
- In means, as the name suggests, the process can be reversed back to its original state or properties.
- It can be possible only if the changes are infinitely small.
- It does not occur in reality, so, it is an ideal process.
Irreversible process
- In means, based on the name, the process cannot be reversed back to its original state or properties.
- All-natural processes are irreversible.
Steady-state process
- Occurs without a change in the internal energy
Thermodynamic Properties
Properties of the thermodynamic system are divided into two types,
- Intensive properties
- Extensive properties.
Intensive properties
- This is the property that is not changed if there is any change in the amount of matter.
- This property is independent of the amount of substance or size of matter.
- Example – density, color, etc.
Extensive properties
- This is the property that totally depends on the quantity of matter or the size of matter.
- It depends on the size or quantity of matter.
- Example – Mass, Volume, etc.
Thermodynamic State
- The thermodynamic state of a system means its conditions, thermodynamic properties, or variables at a specific time.
- All the thermodynamic properties have fixed values at a given state.
Thermodynamic Equilibrium
- Equilibrium means when there will not be any change of any macroscopic property, and the system is totally isolated from the surroundings.
- There will not be any temperature potentials or temperature difference within the system
- There will not be any pressure difference within the system.
- No pressure difference between the system and the surrounding.
- No chemical reaction.
Type of Equilibrium
There are different kinds of equilibrium,
- Thermal equilibrium – means there will not be any temperature difference throughout the system.
- Mechanical equilibrium – it means there will not be any pressure difference throughout the system
- Phase equilibrium – it means the mass of each phase will be in equilibrium.
- Chemical equilibrium – it means there will not be any difference in the chemical composition over time in the system.
What is internal energy?
It is defined as the sum total of kinetic energy and potential energy.
- Kinetic energy comes from the motion of the molecules
- Potential energy comes from the chemical.
- Internal energy means the energy which is due to the motion of molecules.
- It refers microscopic scale for the molecular speed.
Enthalpy
When the measurement of energy to be carried out for a thermodynamic system, the term enthalpy comes into the picture.
- It is the total heat content in a system.
- Internal energy and the product of pressure and volume together constitute enthalpy.
It is written as, h = u + pv, Where,
- h : Enthalpy
- U : Internal energy
- P : Pressure
- V : Volume
Entropy
Entropy is a thermodynamic function that is used to measure the disorders of a system.
- It measures the thermal energy which is not unavailable to work due to disorder.
- Work is associated with ordered molecular action only.
- The entropy of a solid is less than the gas, as molecules in the solid cannot move freely like gases.
What are the Laws of Thermodynamics?
All the fundamentals in thermodynamics are stated in few laws, these are laws of thermodynamics. There are four laws of thermodynamics.
- Zeroth law of thermodynamics
- First law of thermodynamics
- Second law of thermodynamics
- Third law of thermodynamics
Zeroth Law of Thermodynamics
The zeroth law of thermodynamics states that if two thermodynamic systems are each in thermal equilibrium with a third one, then they are in thermal equilibrium with each other. Let’s consider, any three systems,
- System P
- System Q
- System R
Now, if the System P and system Q are in thermal equilibrium and also system P and system R also in thermal equilibrium, then system P, system Q, and system R will be thermal equilibrium.
First Law of Thermodynamics
The first law of thermodynamics deals with the conservation of energy. It states that energy can neither be created nor destroyed, but it can be changed from one form to another.
- If we charge the battery of the mobile, we can use it. The battery is getting energy. However, this energy is coming from electrical power.
- If we put fuel in the car, the car engine will work. Thermal energy stored in the fuel is creating pressure in the cylinder. The piston moves up & down and the crank rotates car moves. So, energy is the same, only it is converted into different forms.
Second Law of Thermodynamics
The second law of thermodynamics deals the entropy. It states in an isolated system; entropy is always increased.
- It means that heat energy is not able to transfer from a lower temperature body to a high-temperature body, without any external energy source.
- Unless power is provided to an air conditioner, it cannot work or it cannot cool.
Third Law of Thermodynamics
The third law of thermodynamics implies that when the temperature of a state approaches to absolute zero, entropy approaches to a constant value.
- The entropy of a pure crystal is zero, at absolute zero temperature.
- The entropy of any system is the measure of the disorder.
Example of the third law of thermodynamics,
- If ice can be able to cool at absolute zero temperature, then the entropy of ice will be zero. However, practically it is not possible.
- If we increase the temperature of ice, it starts to melt and entropy will increase.
- If we increase the temperature a little more, the entire ice will be converted to water and entropy will be increased.
- Further increase in temperature of the water, steam will form. Here, molecules will move faster and entropy will increase further.
Why Does Thermodynamics Require?
Thermodynamics states the behavior of many variables which is subjected to general constraints and common to all materials. These all constraints are expressed in the laws of thermodynamics which helps to know the followings:
- Producing and using a better fuel-efficient system
- Achieving better fuel economy
- Monitoring & optimization of environment impacts
- Minimization of power consumption
- Power conservations
- Analysis of diseases occurring in various biological systems
- Creation of new resources of energy
- Research on renewable energy
- Analysis of extreme human activities
Application of Thermodynamics
The application of thermodynamics is very wide, and few of them are listed,
- Electricity is mandatory in our life and it is produced in power plants using thermodynamic laws.
- All type of engines which used in various cars, trucks, bikes, ships, airplanes, spacecraft, etc. are working on the basis of thermodynamics laws.
- Air compressors, gas compressors, blowers, fans, etc. also run on the basis of thermodynamics law.
- In extreme summer, we use air conditioners and in the extreme cold, we use heaters. These all are based on thermodynamic laws. Apart from air conditioners, refrigerators, cold storages, industrial refrigeration, heat pumps, etc. all are working on the basis of the laws of thermodynamics.
- The study of thermal power plants, nuclear power plants, hydroelectric power plants, renewable energy-based power plants, etc., are based on thermodynamics.
- Renewable energy sources like solar, wind, tides, water waves, geothermal are based on thermodynamics.
- Heat transfer is a part of thermodynamics, which relates to the transfer of heat from one media to another media and the same concept is applied in heat exchangers, evaporators, condensers, coolers, heaters, radiators, etc.
- Even in the thermometer, thermodynamics is applied
- Formation of dew is on the glass is also the application of thermodynamics
Example of Thermodynamics
Let us consider a car. How is it related to Thermodynamics? We all know, to drive a car we must fill the oil. Without oil, we cannot drive our car. Once we fill the oil tank, if we start our car it will move. What is happening here? Why the car move?
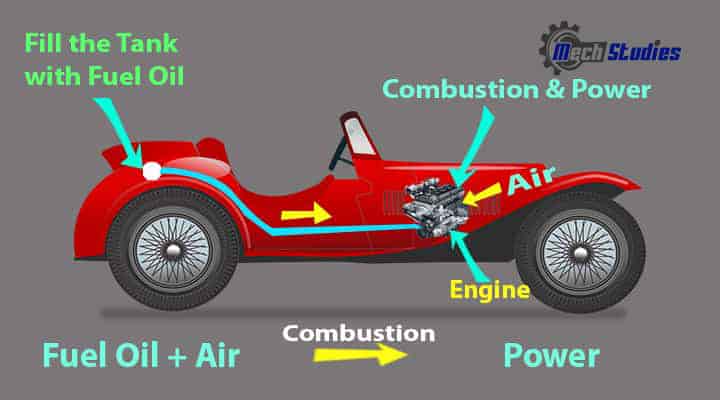
Let me explain, fuel is the source of chemical energy and once we start our car, this energy is converted into heat energy in the presence of air in the engine fuel combustion chamber. Now, this heat energy changes into mechanical energy and transferred to the shaft which rotates the wheels and we move.
Exhaust gas is released from the car. Hence, it is related to heat energy and energy transfer and simply an application of thermodynamics.
Thermodynamics Examples in Daily Life
Whether we are sitting in an air-conditioned room or traveling in any vehicle, the application of thermodynamics is everywhere. We have listed a few of these applications below:
- Different types of vehicles such as planes, trucks, and ships work on the basis of the 2nd law of thermodynamics.
- The three modes of heat transfer work on the basis of thermodynamics. The heat transfer concepts are widely used in radiators, heaters, and coolers.
Limitations of Thermodynamics
Thermodynamics gives details information about heat, work & energy. However, there are a few limitations as well:
- Thermodynamics is concerned with large-scale i.e. macroscopic changes only and it does not specify any internal structure of molecules or atoms.
- Thermodynamics points out the way without any knowledge of time i.e., how long it takes.
- It does not specify the mechanism of the process.
Conclusion
So, finally, we have got a basic idea about what is thermodynamics along with its basic details.

Some really choice blog posts on this site, saved to my bookmarks.